31![cobas®D CT/NO Test and cobas®D CT/NO Test, version[removed]k) Summary Report Roche Molecular Systems, Inc. Pleasanton, CA[removed] cobas®D CT/NO Test and cobas®D CT/NO Test, version[removed]k) Summary Report Roche Molecular Systems, Inc. Pleasanton, CA[removed]](https://www.pdfsearch.io/img/34d423d92264207b3b02ef70fe51e3ad.jpg) | Add to Reading ListSource URL: www.accessdata.fda.govLanguage: English - Date: 2014-06-06 11:54:39
|
|---|
32![cobasID CT/NG Test Section 5: 510(k) Summary 510(k) Summary Report Roche Molecular Systems, Inc. Pleasanton, CA[removed] cobasID CT/NG Test Section 5: 510(k) Summary 510(k) Summary Report Roche Molecular Systems, Inc. Pleasanton, CA[removed]](https://www.pdfsearch.io/img/07df1d9a9180cc9a295c3626ae947da7.jpg) | Add to Reading ListSource URL: www.accessdata.fda.govLanguage: English - Date: 2014-01-14 08:01:16
|
|---|
33 | Add to Reading ListSource URL: www.accessdata.fda.govLanguage: English - Date: 2012-12-06 10:13:11
|
|---|
34 | Add to Reading ListSource URL: www.accessdata.fda.govLanguage: English - Date: 2013-01-09 11:27:41
|
|---|
35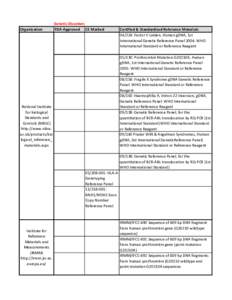 | Add to Reading ListSource URL: wwwn.cdc.govLanguage: English - Date: 2013-06-20 10:04:04
|
|---|
36 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
37 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
38 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
39 | Add to Reading ListSource URL: www.cdc.govLanguage: English - Date: 2012-02-15 09:31:07
|
|---|
40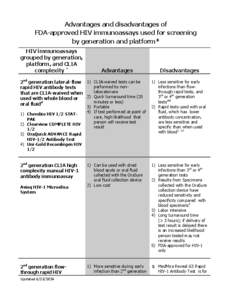 | Add to Reading ListSource URL: www.cdc.govLanguage: English - Date: 2014-06-23 17:12:24
|
|---|